Engineered to Perform Like a Perfect Saphenous Vein
VenoGraft starts with real human vein and transforms it into a durable, immune-invisible biologic conduit.
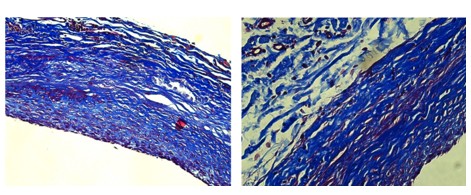
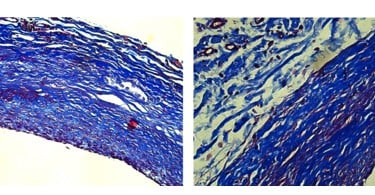
Each step of the process is designed to preserve the geometry and extracellular matrix architecture that make native vein the gold standard in bypass surgery,while eliminating the problems that limit availability and durability.
We begin with ethically sourced human vein from accredited U.S. tissue partners. Only veins with the right size, strength, and structure enter the VenoGraft process.
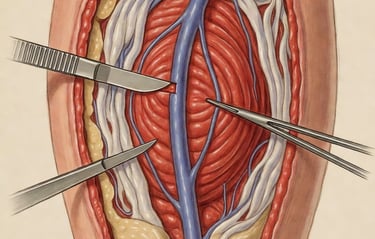
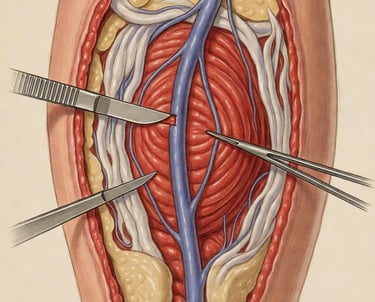
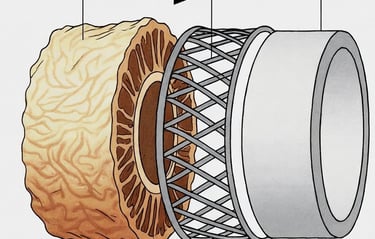
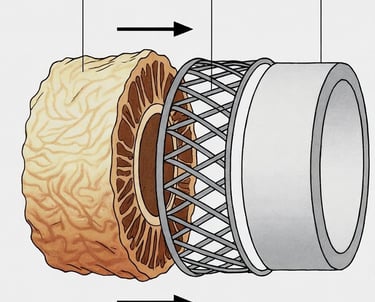
Donor Vein Harvest
Decellularization
Strengthening & Stabilization
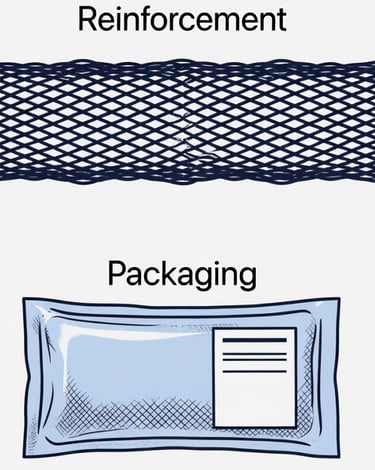
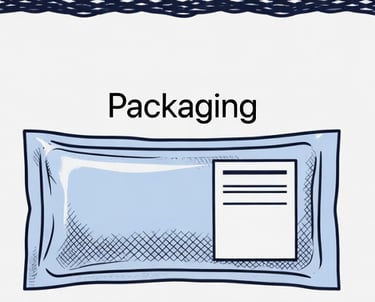
Sterile, Off-the Shelf
Cells and DNA are removed, leaving behind the natural scaffold, that gives it strength and flexibility.
The vein scaffold is strengthened to handle the high-pressure, high-fatigue environment of distal limb bypass.
The final product is sterilized, quality-tested, and sealed for long-term storage, ready when surgeons need it.
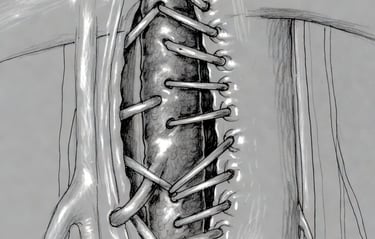
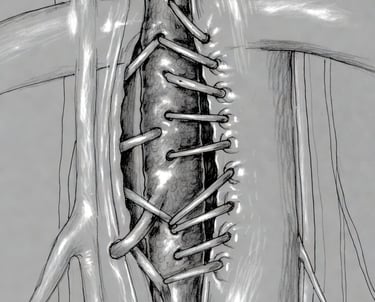
Preclinical Validation
9-month rat Xenobiotic Model ≈ 15–20 human years durability
Human-derived scaffold preserves natural extracellular matrix
Demonstrated resistance to thrombosis and mechanical fatigue
Optimized glutaraldehyde treatment for structural strength
Designed to withstand arterial pressure in lower extremity bypass